SUCCESSFUL MULTINATIONAL CLINICAL TRIALS
Linguistic Validation of Clinical Outcome Assessments
Mitigate risk, speed up time-to-market and ensure global regulatory compliance with specialist linguistic validation of clinical research documentation for sponsors and CROs worldwide.

State-of-the-Art Linguistic Validation
Reduce Time-to-Market & Ensure Regulatory Compliance
Dozens of Therapeutic Areas, Including…
Cardiology
Endocrinology
Genetic Diseases
Infectious Diseases
Neurology
Oncology

Experts in Translating
COAs
ClinROs
PROs/ePROs
ObsROs
COAs/eCOAs
Patient Diaries
Abstracts & Manuscripts

Quality Assurance
Standards
ISO 9001:2015
ISO 13485:2016
ISO 17100:2015
ISO 18587:2017
ISO/IEC 27001:2013
ISO/IEC 27701:2019
Clinical Research In Numbers (2022)
880+
CLINICAL STUDIES
55M+
WORDS TRANSLATED
250+
LANGUAGES
Why Welocalize?
24-48 Hour Quote
Turnaround
Our industry focus has made us experts in knowing exactly what’s
required to localize and validate your clinical documents with accuracy.

Reduced
Translation Time
With a growing network of over 250,000 in-country linguistic
resources, you can expect industry-leading turnaround times.

Rigorous Quality
Methodologies
Dual forward translation, clinician review, and cognitive debriefing ensure instruments are culturally relevant and psychometrically comparable.

Industry-Leading
Accuracy
An industry-leading 7 ISO certifications support our translation and validation processes, hitting all quality assurance standards.
CLIENT SUCCESS
“In order for an instrument to be used in international studies, it must address the same concepts in all languages in order to make it possible to pool data and compare results across countries. Welocalize provides linguistically and culturally accurate translations, which are critical components of pooling data across countries.”
Private Japanese Pharmaceutical
[CASE STUDY] LINGUISTIC VALIDATION
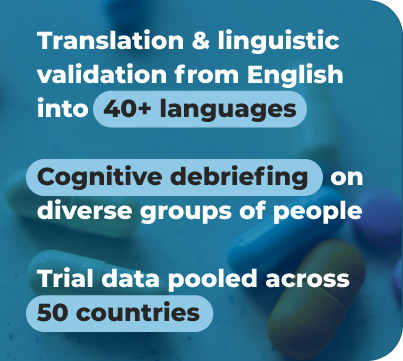
Translation into 40 Language for 50-country Drug Trial
A successful collaboration with a private, billion-dollar Japanese company specializing in several therapeutic areas. We translated ten patient instruments used to measure medication effectiveness into 40 languages for a global drug trial.

Why Linguistic Validation is Important in Clinical Research Translation
Linguistic Validation is the most rigorous translation process in the…
CASE STUDY: Batch Record Translation Under Pressure
Translating registration documents for the world’s second-largest pharma company.