Life Sciences

Transforming Translation in Life Sciences: Rethinking ICR
In the dynamic field of life sciences, the accuracy and…

Revolutionizing Patient Engagement: The Transformative Power of AI in Healthcare
The field of healthcare is rapidly evolving, and artificial intelligence…

Enhancing Diversity in Clinical Trials: The Transformative Role of AI
In today’s fast-paced medical landscape, the call for diversity in…

Biotech Company Streamlines Patent Translation
Global biotechnology company partners with Welocalize to streamline its patent…

Cognitive Debriefing: Transforming Translation in Life Sciences
Welocalize’s Megan Kregel had no idea what linguistic validation was…
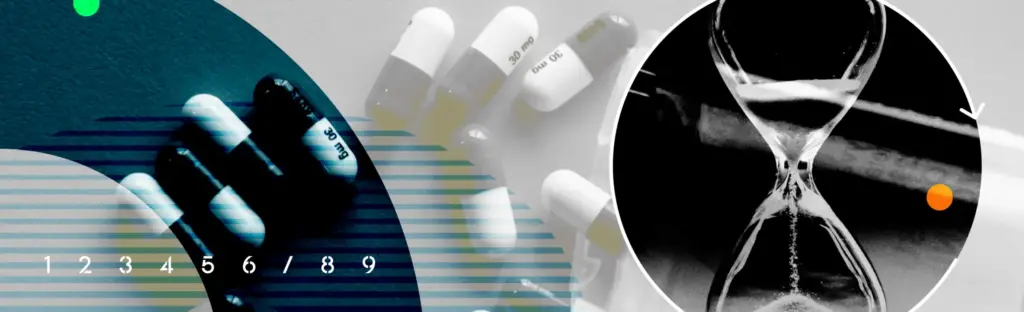
CASE STUDY: Batch Record Translation Under Pressure
Translating registration documents for the world’s second-largest pharma company.

5 Reasons Why ISO 13485:2016 Matters for Medical Device Content Translation
When it comes to medical device content translations, it’s critical…

Using Multimedia in Life Sciences to Educate Global Audiences
From e-learning modules and patient education videos to clinical trial…

Why Linguistic Validation is Important in Clinical Research Translation
Linguistic Validation is the most rigorous translation process in the…

CASE STUDY: Linguistic Validation of PROs for Drug Trial
Japanese pharmaceutical partners with Welocalize in a drug trial across…
 Siobhan Hanna
Siobhan Hanna Erin Wynn
Erin Wynn
 Nicole Sheehan
Nicole Sheehan Kimberly Olson
Kimberly Olson Matt Grebisz
Matt Grebisz Christy Conrad
Christy Conrad Chris Grebisz
Chris Grebisz Dan O’Brien
Dan O’Brien