Human vs. machine | cHAPTER 4
The Human Touch
in Translation

The stakes are always high for localization in the life sciences industry. However, balancing speed, accuracy, and cost is a constant challenge.
While other industries must also balance speed, accuracy, and cost in localizing content, life sciences organizations face unique challenges.
Advancements in AI and neural machine translation (NMT) technologies have significantly improved the quality of machine-generated translations.
Despite AI advancements, human translators remain irreplaceable. Localization requires a nuanced understanding of languages and cultural sensitivity, which AI is not (yet) capable of.
The decision between machine and human translation hinges on balancing speed, accuracy, and cost.
The landscape of translation technologies is constantly evolving. Advancements in AI, machine learning, and NMT will continue to refine the quality and capabilities of machine translation.
Despite AI advancements, human translators remain irreplaceable. Localization requires a nuanced understanding of languages and cultural sensitivity, which AI is not (yet) capable of. Moreover, it is still prone to bias, toxicity, and hallucinations, creating false statements.
Understanding Cultural and Linguistic Nuances
On the other hand, human translators understand the cultural context, idiomatic expressions, and the emotional nuances of language, ensuring translations resonate with the target audience. This allows them to translate content that’s linguistically accurate and culturally appropriate.
Compliance With Regulatory Standards
This is even more critical for life sciences translations, which are subject to strict regulations. Human expertise is essential for ensuring translations meet these requirements and accurately convey critical safety information. This especially applies to patient-facing materials.
In pharmacovigilance, human translators play a vital role in reviewing and refining machine-translated reports, ensuring they meet the stringent regulatory requirements and accurately convey critical safety information across diverse global audiences.
More Accurate Translations
Patient consent forms, drug labels, and other documents must avoid misunderstandings or causing unintended offenses. They must ensure that patients understand and follow what they read correctly. Life sciences organizations should guarantee patient safety by reducing the risk of medical errors from miscommunication and misunderstanding of translated text. It’s not only risk prevention, but correct and proper translations foster trust among patients and higher satisfaction.
Case Study: Global Localization for a Medical Technology Company
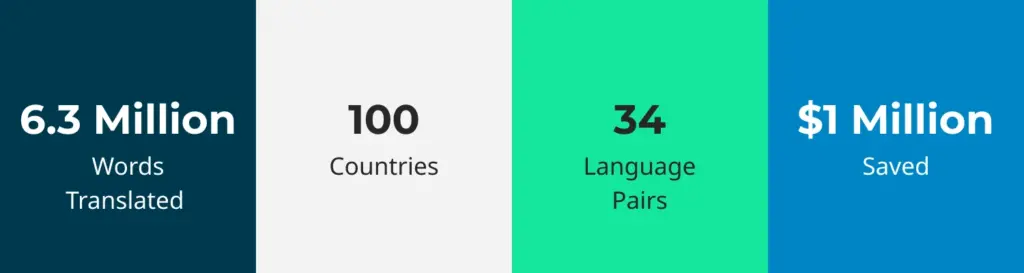
A global medical technology company active in over 100 countries needed an LSP with experience and expertise in the medical devices sector. Some areas for localization included technical documentation, marketing collateral, software UI, and functional hardware testing.
To address the need for accuracy, compliance with regulatory standards, and protection of the client’s intellectual property, Welocalize set up dedicated teams specializing in medical devices across three time zones.
We used advanced translation technology, including machine translation (MT) and translation memory (TM), to translate 6.3 million words across 34 language pairs and conduct functional tests on eight medical devices. As a result, the client saved $1 million.
 Siobhan Hanna
Siobhan Hanna Erin Wynn
Erin Wynn
 Nicole Sheehan
Nicole Sheehan Kimberly Olson
Kimberly Olson Matt Grebisz
Matt Grebisz Christy Conrad
Christy Conrad Chris Grebisz
Chris Grebisz Dan O’Brien
Dan O’Brien